Changes and updates in feed regulation – 2019 overview
Posted 11 February, 2020
Throughout 2019, there have been some interesting changes and trends in the feed additive landscape. In this brief review of the year, we will focus on key updates from EU Regulation and EFSA.

In April 2018, the European Commission (EC) responded to an European Citizens’ Initiative (ECI) to “ban glyphosate and protect people and the environment from toxic pesticides” and requested more transparency around the scientific studies used to support regulated products in the food chain. This led the EC to perform a “Fitness Check” to assess whether the current regulatory framework was fit for purpose. The outcome of the fitness check and public consultation was the creation of a new regulation that amends the General Food Law and covers the agri-food chain to increase transparency in the risk assessment process.
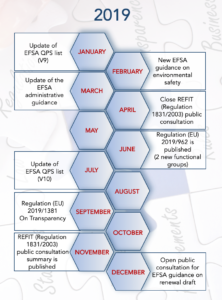
The new Transparency Regulation (Regulation (EU) 2019/1381) amending the Feed & Feed Additive Regulations (among others) was published in September 2019. The new food transparency regulation is expected to cement consumer trust and bring closer authorities and industry. However, it will also be a game-changer for the feed additive authorisation process introducing new habits such as hard bargaining for confidential information or notification of all studies. A public consultation procedure regarding studies performed for renewal dossiers will also be implemented.
So, if you want to waive the new transparency regulation, keep in mind its general application date, March 2021, and remember to include that feed additive application in your 2020 new year’s resolution (Pen&Tec article).
The REFIT (Regulatory Fitness and Performance Programme) evaluation of the Feed Additive Regulation (Regulation (EC) No 1831/2003) was launched in 2017. The Feed Additive Regulation has been in place since 2003 but now faces some changes under the assessment of whether it is ‘fit for purpose’. In December 2018, a public consultation started and lasted 4 months, ending in April 2019 (Pen&Tec article) whereby 110 replies from a range of company & business organisations, individual citizens, public authorities and other groups were received (link). The results of the questionnaire revealed many interesting viewpoints. Opinions on the general safety and efficacy of feed additives and their centralized assessment seemed to be unanimously positive. Most of those who took the survey believed that the current Regulation is still fit for purpose.
Current Feed Additive Regulation protects safety, but does it allow for innovation?
Only a minority of responders considered the Regulation sufficiently flexible to adapt to new scientific and technical developments or did not see the need for new functional groups.
In June, an amendment to Annex I of the Feed Additive Regulation establishing two new functional groups was published (Regulation (EU) 2019/962). The new groups were:
1(o) “Other” (category: technological additives): The functional group “other” allows the applicant to propose a function of the additive. Up to 12 June 2019, this functional group just existed for zootechnical feed additives.
4(e) “Physiological condition stabilisers” (category: zootechnical additives). This new category includes feed additives that may help animals to keep a good physiological condition, contribute to animal welfare, favourably affect the animal resilience to stress factors or to support their wellbeing in certain situations.
It is indeed of great importance for the sustainability of the industry, that the EU legislative framework provides room for innovation but do these new functional groups truly open the door for greater innovation? (Pen&Tec article). At the Feed Info conference held in Amsterdam in September, during our regulatory workshop Pen & Tec asked the audience if they thought that the new functional groups allow for greater innovation in the industry. In total, 89% of the audience believed that the creation of the new functional groups will help create more innovation (Pen&Tec article).
Throughout 2019, 40 new feed additive authorisations were granted in the EU, reflected in the 12 editions of the EU Register of Feed Additives (link). 7 were technological additives, 10 sensory additives, 21 nutritional additives and 16 zootechnical additives. No new additives were authorised in the coccidiostats and histomonostats group. The feed material catalogue (Regulation (EC) No 68/2013) was not updated this year (only a typo in the Dutch version was amended) but it is currently under revision and an amended version will be published during 2020.
Currently, only authorisations for feed additives under the zootechnical and coccidiostat groups are holder-specific whilst feed additives belonging to other groups do not receive the same market protection upon authorisation. This means that once these products are authorised, any company can sell the same product providing they meet the authorisation requirements for that product potentially dissuading investment in new novel products from these feed additive categories by companies. By making all authorisations holder-specific, an environment which favours innovative ideas may be generated and a rise in investment in this area may be observed. Only time will tell whether such changes will be considered.

Meanwhile in EFSA, after its own “REFIT” evaluation, the update of the FEEDAP Guidance Documents (link) continued throughout 2019. EFSA published the new environmental safety guidance (link), an updated version of the administrative guidance (link) and the guidance on renewals draft (which was open for public consultation until 26 January 2020) (link). EFSA has now posted a technical statement on the requirements for the whole genome sequence analysis of microorganisms intended for use in the food chain (link), which is open for public consultation until 28 February 2020 and hopefully witness an update on the left-behind user safety guidance (published in early 2012) in the new year.
2019 brought us also two QPS list (Qualified Presumption on Safety) updates (No. 9 (link) and No. 10 (link)) covering taxonomical units submitted between April 2018 to March 2019. A total of 95 notifications were received (73 were feed additives). Out of the total, 49 were already QPS and 35 belonged to microorganisms excluded from the QPS qualification (filamentous fungi, E. coli…). From the remaining 11, only Komagataeibacter sucrofermentans, Corynebacterium ammoniagenes & Euglena gracilis were proposed for the QPS list (production purposes). Additionally, the QPS status of Corynebacterium glutamicum was extended to other production purposes and Pediococcus dextrinicus nomenclature changed to Lactobacillus dextrinicus.
To conclude, 2019 was a year of changes towards making our food chain safer and more transparent embracing industry and citizens’ needs.
By Ana Merino Tejedor, Regulatory Affairs Manager & Caroline Idowu, Regulatory Affairs Associate
Pen & Tec Consulting
