FEED ADDITIVE RENEWALS
Under the new transparency regulation, the procedure for feed additive renewals has changed.
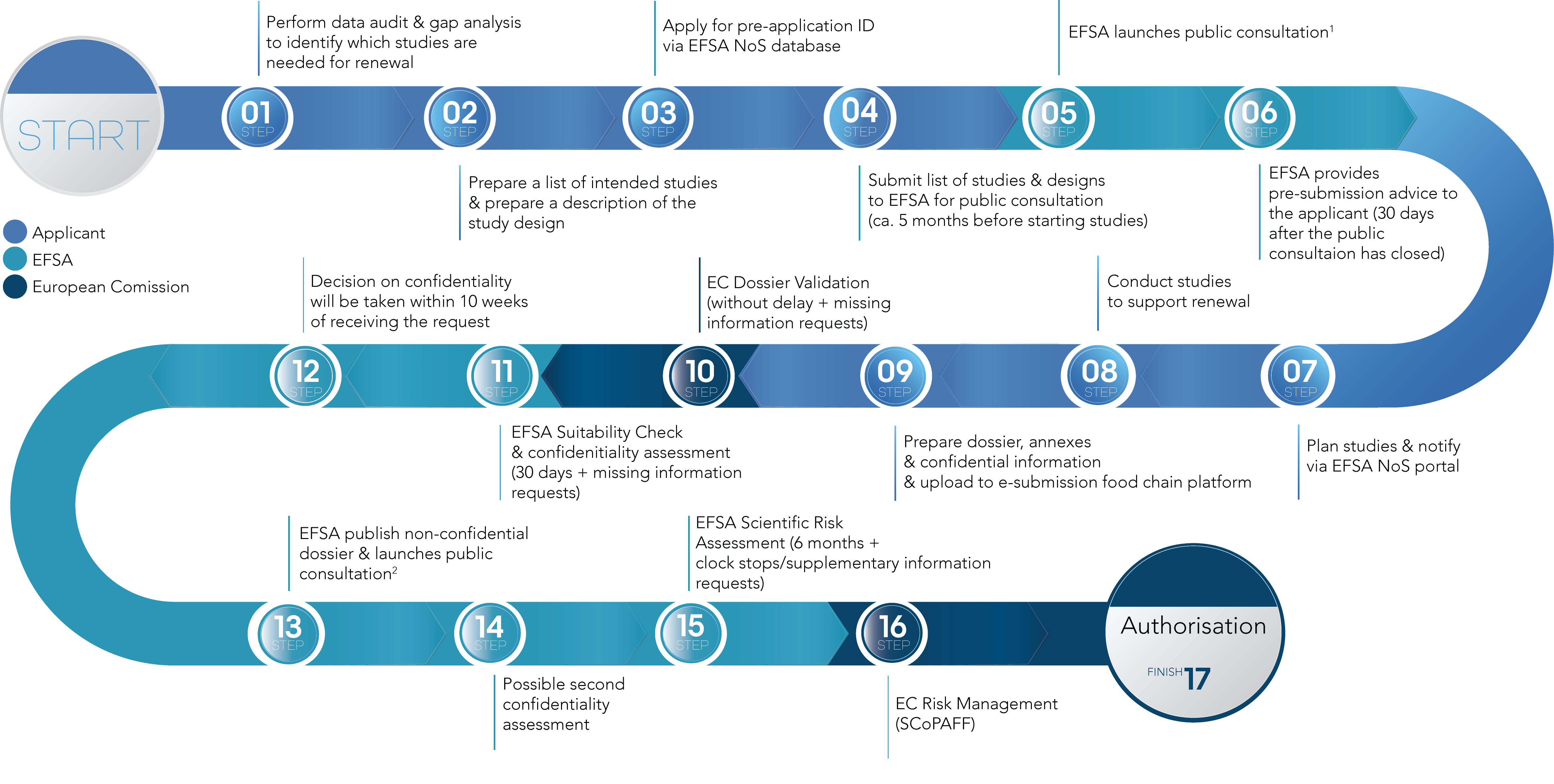
Now there is a pre-notification and public consultation step that needs to be performed before the renewal dossier can be submitted. At the beginning of the renewal process, the applicant needs to notify EFSA regarding the studies they plan on conducting. EFSA will then launch a public consultation with third parties on the planned studies. After the public consultation, EFSA will provide tailor-made advice to the applicant on the content of the renewal dossier as well as on the design of the studies.
The new procedure and timelines are presented in the figure below:
Notes:
- Feed additive & plant protection product (PPP) authorizations are valid for 10 years and must be renewed
- The renewal dossier should be sent 1 year before the expiry date of the authorisation
- Once the Transparency regulation applies, it is advisable to start the renewal process 2-years before the expiry date of the authorisation!
1 The public consultation is open for 3 calendar weeks. In the case of PPPs, EFSA will provide renewal pre-submission advice with the participation of rapporteur Member State(s) and the co-rapporteur Member State.
2 The non-confidential version of the dossier will be released after validation and in parallel EFSA will start the assessment of the confidential version (or confidentiality assessment).
Pen & Tec´s regulatory experts can help you to navigate your way through the new EU regulation on transparency to minimise delays, confusion and to ensure you get your product to market in a cost effective and time effective way. We can provide strategic advice and support for all steps of the application process including support for all pre-submission activities, notification of studies, preparing for and obtaining pre-submission advice from EFSA and advice on how to manage confidentiality. We can also assist with preparing and submitting the dossier fo you.
If you need guidance or have any questions, feel free to contact us and we will be happy to help.