New EU regulation on transparency: what does it mean for your food and feed approval?
Posted 11 October, 2019
In April 2018, the European Commission (EC) responded to a European Citizens’ Initiative (ECI) to “ban glyphosate and protect people and the environment from toxic pesticides” and requested more transparency around the scientific studies used to support regulated products in the food chain. This led the EC to perform a “Fitness Check” to assess whether the current regulatory framework was fit for purpose.
The EC issued a proposal for the new Regulation in 2018, which was subject to an extensive open public consultation to citizens, national authorities and “stakeholders”. The stakeholders that took part were represented by variety of sectors and expressed various aspects that could be improved including:
- Earlier access to the industry studies ensures a higher transparency,
- Importance of safeguarding confidentiality and intellectual property rights,
- More clarity needed on what will apply as confidential information,
- Verification on the quality of industry studies, CROs and laboratories involved,
- Positive impact of EFSA’s pre-submission advice to industry applicants,
- Increase capacity for more public resources to finance studies,
- The need to tackle potential negative impacts of consultations on studies submitted on the length of the assessment processes,
- Improve risk communication,
- Further involvement and incentives of Member State (MS) authorities and experts in EFSA’s activities ensuring excellence although maintaining the separation between risk assessment and risk management.
The outcome of the fitness check was the creation of a new transparency regulation, Regulation (EU) 2019/1381 that amends the General Food Law (2002) and covers eight legislative acts dealing with the agri-food chain to increase transparency in the risk assessment process affecting the following areas:
- GMOs (cultivation and for Food/Feed uses)
- Feed additives, smoke flavourings
- Food contact materials
- Food additives
- Food enzymes and flavourings
- Plant protection products
- Novel foods.
The main objectives of the Regulation will be to improve and clarify the rules on transparency, particularly related to:
- Scientific studies used to support regulated products in the food chain
- Increasing the reliability, objectivity and independence of the studies
- Improving the governance and strengthening the scientific cooperation and involvement of Member States (MS) in EFSA
- Addressing the limitations affecting the long-term scientific capacity of EFSA and its ability to maintain a high level of scientific expertise across the different areas of the agri-food sector, taking into account the related financial and budgetary aspects
- Developing a more effective and transparent risk communication with the public in collaboration with MS.
Although the new Regulation was published on 6th September 2019 in the Official Journal, it will not apply until the end of March 2021 – be ready!
What are the main changes that will affect you?
Pre-submission advice
A potential applicant can ask for a pre-submission meeting for “advice” on the potential authorization application, rules applicable and the content required.
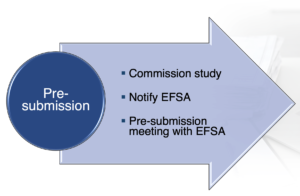
Figure 1. Pre-submission procedure under the new regulation
Studies and confidentiality
- All studies will need to be notified – EFSA will request a mandatory pre-application submission notification for all studies to be conducted by laboratories and potential applicants, including the title, scope, testing facility, starting and planned completion dates.
- A database of all commissioned studies by the industry will be available and managed by EFSA.
- All personal data will remain confidential unless it is strictly necessary to ensure transparency.
- All non-confidential studies and information supporting an application to be assessed by EFSA are to be made public automatically when the application is validated.
- EFSA firstly, will publish the non-confidential version supplied by the applicant, then proceed to assess the confidential information issuing a decision, if the applicant disagrees they will have the possibility to withdraw the application or state and argue its views within two weeks of the date on which the EFSA decision was notified. After the final decision the full set of studies is published (except the agreed confidential data).
- Applications will not be considered valid when,
- Supported by studies that have not been previously notified, unless a valid justification is provided. An application may be re-submitted when the applicant notifies those studies to EFSA. The assessment of the validity will be suspended until six months after the notification of those studies.
- studies that have been notified are not included in the application, unless a valid justification is provided. The risk assessment will be suspended until six month after the submission of all data of those studies.
- Controls, including audits, will be carried out on the compliance of laboratory/studies with standards.
- EC could ask EFSA, in exceptional circumstances, to carry out their own studies for verification purposes.
EFSA will launch a consultation to stakeholders and general public on submitted studies (non-confidential version) to identify whether other relevant information is available on the substance at stake.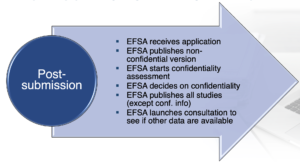
Figure 2. Post-submission process under the new regulation
Renewals
- For renewals, a pre-notification by the potential applicant for planned studies has to be submitted,
- EFSA will then launch a consultation to third parties on the planned studies and will provide tailor-made advice to the applicant on the content of the renewal dossier as well as on the design of the studies.
Involvement of Member State experts
- EFSA’s management board will include representatives of all MS that will have the right to vote, each MS shall nominate a member and an alternate member based on their experience and expertise,
- The appointment of experts to EFSA’s scientific planes will be made from a pool of nominations put forward by MS where the Management Board will have the final decision,
- Strict independence criteria will be maintained to ensure excellence,
- EFSA commitment of scientific panels’ work to be better organised.
Risk communication
- Improve coordination between risk assessors and risk managers to ensure better communication to stakeholders and general public,
- Foster public understanding of the risk analysis to enhance confident in its outcome.
What about confidentiality?
The new regulation has made provision for confidential information & confidentiality will be guaranteed if the information is commercially sensitive & if making the information available to the public would significantly damage the applicants´ commercial interests. Types of information & data that will be protected includes:
- The manufacturing process, technical and industrial specifications, specification on impurities (as long as there are no safety concerns),
- Any methods of analysis that have been developed internally,
- Personal data,
- Study plans or protocols for efficacy studies that are performed to demonstrate an additives intended use & this covers all feed additive categories & functional groups,
- IP, data exclusivity & data protection will be guaranteed.
One other important change is that when the regulation enters into force, it will be EFSA that decides on which data will be grated confidentiality instead of the EC, who is currently responsible.
What does the industry think about the impact of the new regulation?
We asked the audience at the Feed Info Feed Additives Europe event in Amsterdam in September 2019 whether they thought the new EU regulation on transparency would be good or bad for industry. In total, 34% of the audience thought that the new regulation would be good for industry, while 20% believed that it will negatively impact our industry. The remaining 46% did not know whether it would be good or bad as there is still a lot of uncertainty over the process & believe that we will have to wait & see how strict EFSA is with granting confidentiality.
We then asked the audience if they thought that the tailored advice provided by EFSA on the content of renewal dossiers & study design that will be applicable once the new regulation on transparency enters into force will be a good thing. In total 68% believe this will be good for applicants, while 19% voted “don´t know”. Interestingly, 13% of the audience believe this will not be good for applicants as the outcome of the consultation will be made available on the EFSA website.
Looking to the future….
The new regulation on transparency will definitely have a huge impact on the current regulatory framework for the agri-food sector and there will be challenges for the industry and regulators to adapt to the new approach. The European food safety chain is moving forward to maintain and improve its scientific excellence, ensure further transparency, reliability and objectivity but needs further protection for innovators and creativity, where the current framework lacks the flexibility to adapt to scientific and technological development.
The new transparency regulation will be a game changer for our industry & 27th March 2021 is a key date to put in your diaries.
For more information on the EU regulation on Transparency, please feel free to contact us: info@pentec-consulting.eu & sign up to out free online webinar.

By Cristina Martin Jimenez, Regulatory Affairs Associate
Pen & Tec Consulting
