NOT SURE IF YOUR PRODUCT IS A NOVEL FOOD?
Foods or food ingredients that have not been consumed to a significant degree in the EU before 1997 are considered “novel,” under Novel Food Regulation (EU) No. 2015/2283.
Examples of novel foods or novel ingredients are:
- Newly developed foods e.g. alternative-proteins from cell-based meats, insect proteins, novel plant proteins
- New products/ingredients produced from existing food (e.g. coffee flour, coriander seed oil)
- Food produced using new manufacturing processes ( or novel technologies (e.g. nanomaterials)
- Food that is traditionally eaten outside of the EU
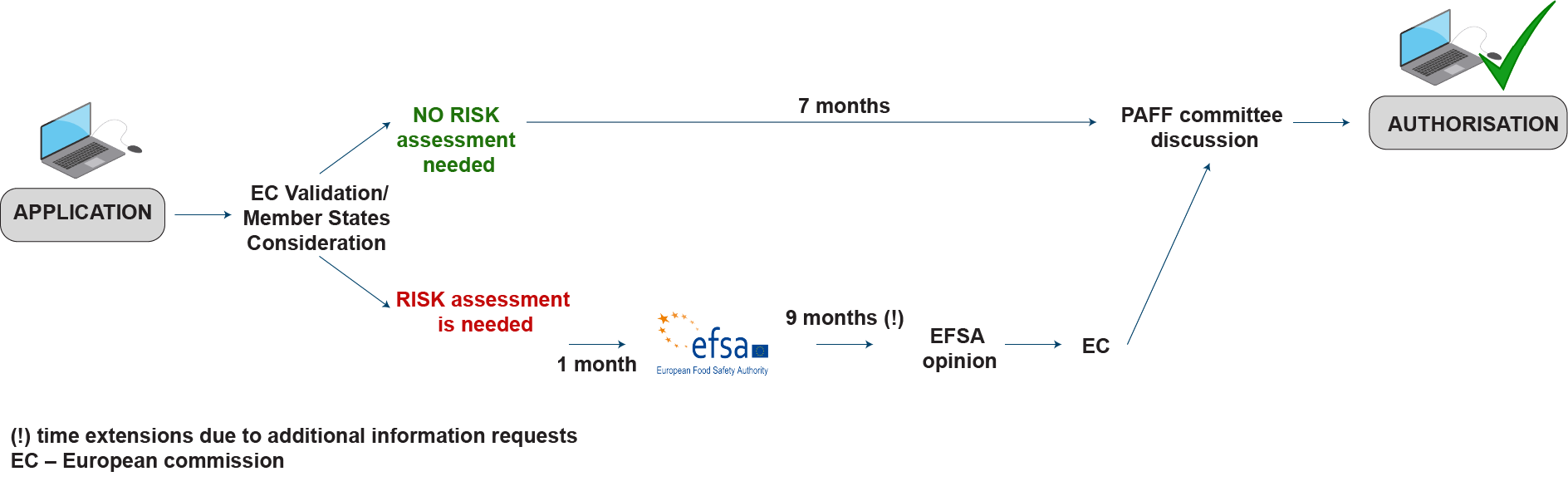
Novel food and novel food ingredients cannot be sold on the EU market unless they have undergone a thorough novel food safety assessment to prove that the food is safe for human consumption. Applicants must provide evidence that their product is safe. To do this, they need to submit a novel food dossier to the European Commission (EC). Once the dossier has been received, a scientific safety assessment is performed by the European Food Safety Authority (EFSA).
Once the novel food has been approved by the EC, it is listed in the EU Union List of Novel Foods (Regulation (EU) 2017/2470).
For more information on UK authorisation, please visit this web page.
An outline of the novel food registration process is presented below:
(!) time extensions due to additional information requests; EC – European Commission; EFSA – European Food Safety Authority; PAFF Committee – Standing Committee on Plants, Animals, Food and Feed
NOT SURE IF YOUR PRODUCT IS NOVEL?
Contact us if you have questions or need regulatory assistance.