ALTERNATIVE PROTEIN REGULATIONS – ALL YOU NEED TO KNOW
In today’s world consumers and business become more concerned about the impact of the animal-based protein on the environment. Every year, the global amount consumed keeps increasing, especially in developing markets. However, the increased production of conventional protein takes its toll on the environment & the increased consumption also leads to additional risks for human health.
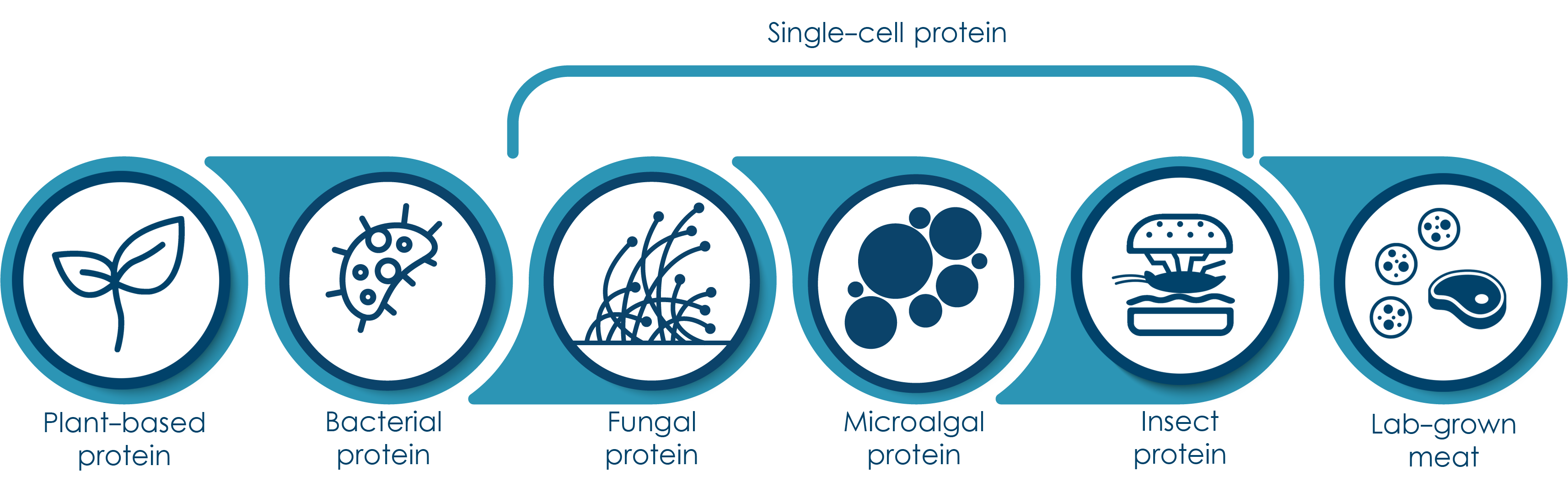
Thus many business are actively working on sustainable protein alternative and many companies are interested in marketing alternative protein ranging from plant-based protein isolates, single-cell protein & insect protein to lab-grown products developed from cultivated animal cells.
Figure 1. Different types of alternative protein
Alternative proteins have the potential to fall under the definition of a novel food in the EU. Novel foods are defined as foods that had not been consumed to a significant degree by humans in the EU before 15 May 1997. All novel foods or food ingredients require a safety assessment and a pre-market authorization before they are included in the Union list and can be sold in the EU.
The application process starts with the submission of a novel food dossier to the European Commission (EC) by the applicant. The EC has 1 to 3 months after the receipt of the application to perform a validation check, in which it will check whether the application is complete. The EC makes the application available to the Member States (MSs) & requests EFSA to perform a risk assessment. EFSA will, on its turn, perform their own validation check & will evaluate the application within 9 months from the date of receipt of a valid application. EFSA will then publish their opinion on the application & forward it to the EC, the MSs & the applicant. The EC has then 7 months to publish a draft implementing act authorising the novel food. During meetings of the Standing Committee on Plants, Animals, Food & Feed (SCoPAFF), a decision will be made to adopt EFSA’s scientific opinion on the novel food.
Is your business interested in commercialising alternative protein? Do you need help navigating through the complex regulatory landscape in the EU?
Pen & Tec can assist you:
Besides the EU, Pen & Tec also has experience in other geographies, such as the UK, US, Canada and Singapore.
Are you interested in bringing your alternative protein product into the EU? You might want to check our regulatory guidelines on “Regulatory approval for alternative proteins in the EU – steps to success” – its free! You can download it by clicking the button on the right side on this page.
HAVE A SPECIFIC QUESTION REGARDING YOUR PRODUCT?
Contact us if you have questions or need regulatory assistance.
Please fill in the form to download your free copy of the regulatory report. It will be sent to your mailbox. In case you didn’t receive the e-mail – please check your junk folder or contact support@pentec-academy.com