EFSA stakeholder workshop on nanoscience and nanotechnology – what’s new?
Posted 5 April, 2019
On 1-2 April, 2019, the EFSA Stakeholder workshop took place on nanoscience & nanotechnology, with focus on EFSA’s Guidance on human and animal health aspects (Part 1) of the risk assessment of nanoscience and nanotechnology applications in the food and feed chain.
Pen & Tec regulatory affairs associate, Mikel Goñi, attended the workshop in Parma, Italy and has put together a summary of the key information discussed at the event.
 During EFSA’s, stakeholders had the chance to interact with EFSA and provide their feedback in relation to chapters 3 to 6 of EFSA guidance on risk assessment of the application of nanoscience & nanotechnologies in the food and feed chain: Part 1, human and animal health.
During EFSA’s, stakeholders had the chance to interact with EFSA and provide their feedback in relation to chapters 3 to 6 of EFSA guidance on risk assessment of the application of nanoscience & nanotechnologies in the food and feed chain: Part 1, human and animal health.
From the stakeholders’ point of view, the guidance is very comprehensive and easy to follow, but uncertainty is evident throughout the whole guidance which may have consequences for applicants.
Test Guidelines and Guidance Documents for “traditional” chemical substances can currently be applied to nanomaterials, but generally there is no specific information or requirements regarding nano-testing.
A case-by-case scenario is foreseen & the uncertainty of this presumption could result in unnecessary costs. This is why stakeholders not only require more defined test methods, but also need better defined guidance.
The “Malta Initiative” arose to develop & amend these guidance in order to fulfil regulatory requirements for nanomaterials. This initiative is expected to bring up adapted OECD guidelines with suitable “nano-methods” in the upcoming years. Nevertheless, this will be a long process (2-8 years) and at the moment, searching for a proper method could remain a frustrating topic for applicants.
Is my product a nanomaterial?…. Size matters!
A current “hot” topic in the industry is the question of what is defined as “nano” and “non-nano”. Industry stakeholders would like to see the guidance change from a “nano guidance” to a “size-dependent guidance”.
The NanoDefine decision-flow scheme will most probably be taken out and we should expect to see changes on what is defined as “nano” & “non-nano” in the near future.
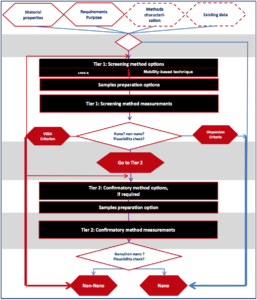
Figure 1. Appendix A. NanoDefine decision-flow scheme
In addition, stakeholders asked for clarity relating to product characterisation demands. Therefore, EFSA will most likely introduce changes in Table 1 of the guidance for easier material characterisation. This table will be redefined in a more mandatory and user-friendly way.
Why is characterisation of nanomaterials so important?
The EFSA Scientific Committee has declared that, at this stage, gathering data is very important for nanomaterial evaluation. Nano-toxicology is considered to be a “young discipline”, and a different safe approach may apply depending on nanomaterials’ size & batch to batch variation (Section 4 of the guidance).
Hazard identification can be related to the typical “small particle behaviour” and it is also important that there is a complete correlation between the material produced and the one tested.
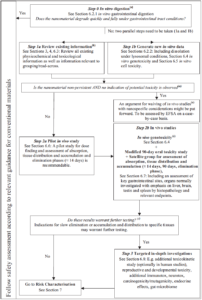
Figure 2. Stepwise framework for nano-related hazard identification and characterisation in food/feed.
What should we expect in the upcoming years?
Uncertainty & expression for greater clarity from industry stakeholders may lead to further changes to not only this nano-guidance, but also to other EFSA guidance.
For example, there are no mentions of or specifications for feed additive applications under the scope of this guidance in any of EFSA scientific guidance for feed additives. Therefore, amendments to general feed guidance might be found in the near future in relation to nanomaterials.
Moreover, the European Commission is about to publish a mandate regarding nanomaterials in the near future, but we will have to be patient to see what this mandate states.
Do you need Regulatory assistance on nanoscience?
If you have any questions, please do not hesitate to get in touch with Pen & Tec as we would be very happy to help!
Bibliography:
EFSA Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA Journal 2018;16(7):5327, 95 pp. https://doi.org/10.2903/j.efsa.2018.5327
By Mikel Goñi
Regulatory Affairs Associate, Pen & Tec Consulting
Mikel Goñi García-Falces holds a bachelor’s degree in Veterinary Medicine (DVM) from the Autonomous University of Barcelona (UAB) & an interuniversity Master of Science degree in Aquaculture (Universitat de Barcelona, UAB, & Universitat Politècnica de Catalunya). During his master’s thesis at the veterinary pharmacology department, he validated analytical methods for the quantification of several antibiotics in the plasma of four different fish species using High Performance Liquid Chromatography (HPLC) and under Good Laboratory Practice (GLP) procedures. Mikel is also experienced in marine animal health and has completed internships in different countries such as Spain, Finland & The Netherlands. Mikel joined Pen & Tec in 2018 and is currently working in different feed additive & novel food applications.
