Species extension for existing feed additive authorisations: Updated EFSA guidance brings great advantages to companies
Posted 21 September, 2018
Many feed additive companies have welcomed the new EFSA guidance on efficacy, which entered into force on 1st September 2018 because the changes bring promising advantages to those considering to broaden their product portfolios. By offering the opportunity to extrapolate existing data to other target species and by reducing the number of required efficacy studies, the updated guidance allows companies to maximise return on investment by extending to other species while, at the same time, working towards the 3 Rs (reduce, refine and replace animals used in scientific studies). The new guidance on target animal safety, which entered into force on 1st May 2018, also allows extrapolation of safety data between physiologically similar species.
At Pen & Tec, we are offering companies species extension bundles, where we can help you to extend your current feed additive authorisation to other target species. In order to take advantage of the species extension, companies can use their existing data to apply for an update to their existing authorisation.
For example, the extrapolation of recent data compiled for certain animals can now be extended the following species:
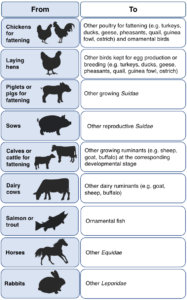
With these new updates helping EFSA work towards taking the target “3R’s” approach in the industry, Pen & Tec want to help companies make the most of this positive change and will be happy to assist your company in any way that we can. Get in touch with us: info@pentec-consulting.eu
