Is it easier to register feed additives in the EU or the USA…..? What did the audience at Feed Additives Americas think?
Posted 21 November, 2019
On the 14th November 2019, Dr Hannah Lester, Scientific Director at Pen & Tec delivered an interactive lunchtime workshop at the Feed Additives conference in Miami.

During the workshop we discussed:
- Contrasts between the US & EU feed additive regulations
- Comparison of EU/US data requirements
- What are the key challenges in terms of regulatory approval
We kicked off the workshop by asking the audience whether they thought achieving a feed additive approval is more difficult in the EU than in the US? Around 80% of the audience believed that it is tougher to get a feed additive authorized in the EU compared to the USA due to a rigid regulatory framework & demanding data requirements.
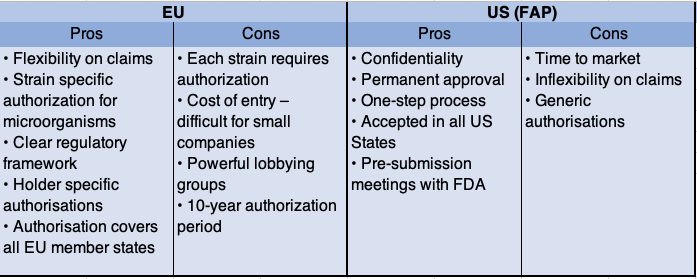
Hannah went on to explain the key differences between the EU & US highlighting that in the EU there are holder-specific authorizations for zootechnical feed additives & coccidiostats, where all feed additives are generic in the US. In addition, in the EU there are clear feed additive categories & functional groups, which brings clarity & there are also guidance documents produced by the European Food Safety Authority (EFSA) to help applicants design & conduct studies. In the US, the FDA is very tough on claims, and historically production & performance claims have not been permitted. However, in the EU, there is far greater flexibility on claims.
We asked the audience if they thought that the EU “holder-specific authorisation” & types of claims encourages innovation & more protection for feed additive businesses compared to the USA? Interestingly, over 70% of the audience thought the EU encourages innovation, but only for large companies that can afford the registration.
Hannah went on to compare the data requirements between the EU & USA (Food Additive Petition (FAP) route) & pointed out that:
- The data set required for US/EU new authorisations is fairly similar
- Main differences: in the EU there are additional requirements for microorganisms, user/worker safety & efficacy studies
- Time to market & cost roughly the same.
- Ideally, design the studies to be both EFSA/FDA compliant as there are a lot of data that can be used in each dossier.
Finally, Hannah asked the audience to work in groups to discuss the key regulatory issues in the EU & USA. These are summarized below:
For more information on any of these topics, or for a copy of the workshop presentation, please email us: info@pentec-consulting.eu
 By Hannah Lester, Scientific Director
By Hannah Lester, Scientific Director
Pen & Tec Consulting
