How will the EU Transparency Regulation affect feed & food regulated products?
Posted 05 May, 2021
The Transparency Regulation (1, officially Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019) that applies from 27th March 2021 will impact the risk assessment performed by EFSA for products in the food chain that require an authorisation, such as feed and food additives, novel foods, smoke flavourings, food flavourings, food enzymes, food contact materials, and genetically modified feed and food, among others. Additionally, the regulation also applies to plant protection products registrations (pesticides) and the Directive 2001/18/EC on the deliberate release into the environment of genetically modified organisms (GMO). This regulation was created in response to the European Citizens’ Initiative “Ban glyphosate and protect people and the environment from toxic pesticides” and, according to the European Commission (2), it is aimed at:
- Increasing transparency of the EU risk assessment in the food chain
- Strengthening the reliability, objectivity and independence of the studies used by EFSA
- Revisiting the governance of EFSA in order to ensure its long-term sustainability
The main changes that the Transparency Regulation will introduce in the General Food Law (3) are summarised below in Figure 1 – all requirements will apply equally to feed and food products.
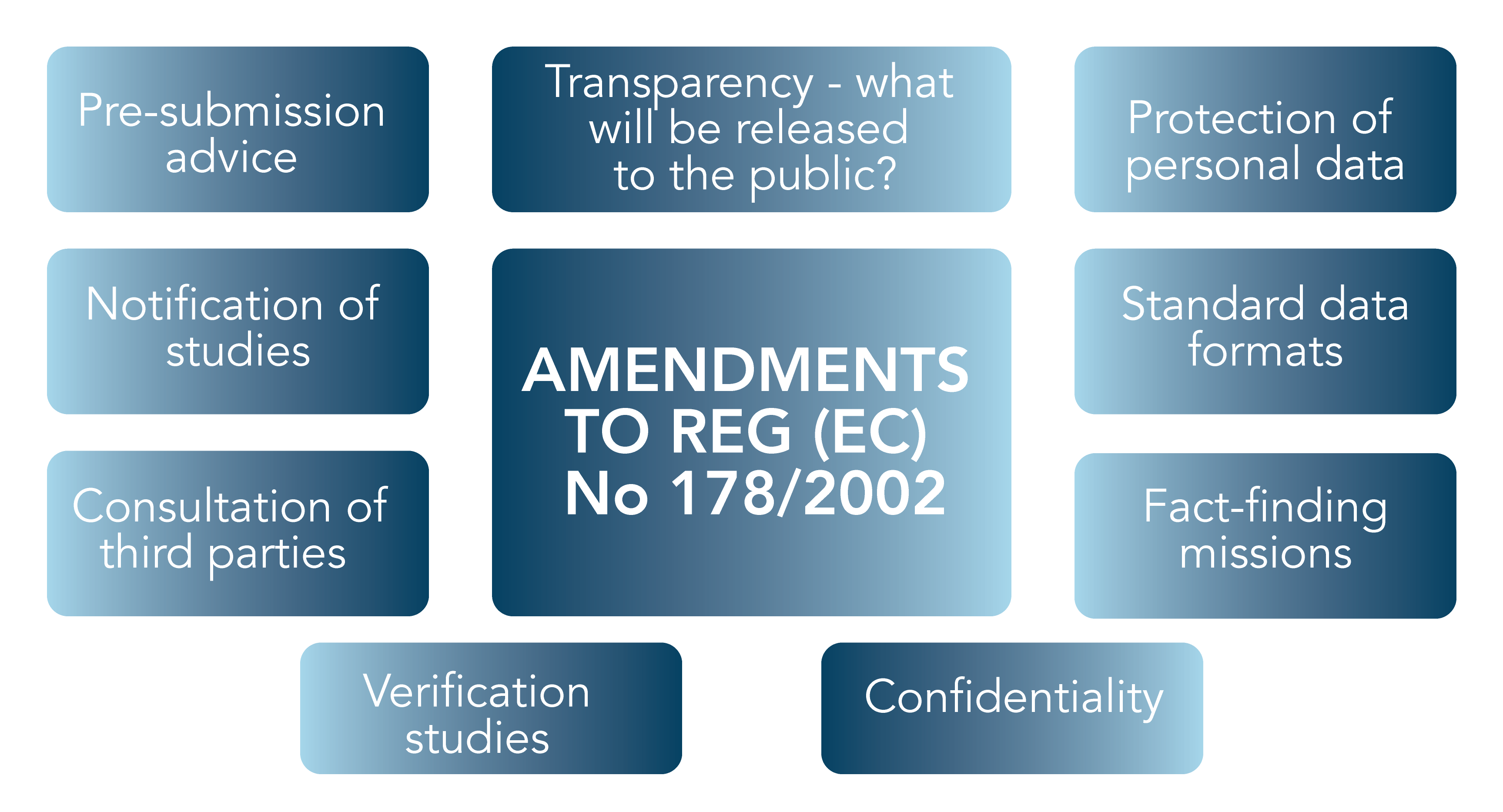
Figure 1. New requirements introduced in Regulation (EC) No 178/2002 (General Food Law)
PRE-SUBMISSION ADVICE FOR NEW APPLICATIONS (ARTICLE 32A)
All applicants intending to register a new feed or food product may request pre-submission advice from EFSA regarding the following points:
- Which are the rules applicable for the application considering the product characteristics and intended use
- The content required for the dossier
The pre-submission advice will be requested and received via written communication using the Notification of Studies Database System (4,5) but, if considered necessary, it could also be provided via teleconference or in a face-to-face meeting with EFSA.
NOTIFICATION OF STUDIES: DATABASE OF STUDIES COMMISSIONED FOR A FEED OR FOOD APPLICATION (ARTICLE 32B)
EFSA will establish a database of studies (4,5) commissioned by a Food Business Operator (FBO) for an application. In this database, applicants or their representatives (such as consultants or partners) and contract research organisations (CRO) will have to notify the following information as soon as it is available:
- Title of the study
- Scope
- Laboratory/CRO name (notified by the applicant)
- FBO name (notified by the laboratory/CRO)
- Study planned start and end date
The notification obligation also applies to CROs located in 3rd countries with trade agreements with the EU – initially Iceland, Liechtenstein, and Norway. If studies are not notified properly, the assessment process could be penalised as follows:
- If the study is not notified to EFSA but it is included in the dossier (and there is no proper justification available): the application can be resubmitted after notification but the assessment of the validity of the resubmitted application will start 6 months after the notification
- If the study is notified but not submitted to EFSA in the dossier (and there is no proper justification available): the application can be resubmitted including all studies notified but the assessment of the validity of the resubmitted application will start 6 months after the submission of the pending information
- If a notified study is submitted partially to EFSA (e.g. there is missing data in the report with no proper justification available): the assessment of the validity of the application will start 6 months after the submission of the pending information
RENEWAL PROCESS: CONSULTATION OF 3RD PARTIES FOR AUTHORISATION RENEWAL (ARTICLE 32C-1)
For products such as feed additives and pesticides, applicants must apply for a renewal of the authorisation after 10 years. When a renewal dossier includes new studies (i.e., studies not assessed by EFSA in the original application for authorisation), applicants must first notify EFSA of the list of studies intended to be performed. For each study included in the list, applicants will need to provide the following information through the Notification of Studies Database (4,5): study title, name of applicant, study scope (study intended area, study type, study objective, test item), study guideline to be followed (if applicable) or study design description (including the study hypothesis). This information will be released to the general public for consultation, and EFSA will take into consideration the comments received when providing advice to companies regarding the content of the application and study design.
CONSULTATION OF 3RD PARTIES AFTER DOSSIER SUBMISSION (ARTICLE 32C-2)
Applicants will need to prepare two dossiers for EFSA – a confidential and a non-confidential version. The latter will exclude all the information applicants want to claim as confidential and will be released to the public for consultation. With this procedure EFSA is seeking to identify whether other relevant information (e.g. scientific data, studies) for the assessment is available. Once the scientific assessment is concluded, EFSA will publish the outcome of this call for data in addition to its scientific opinion. It is important to note that if the authority rejects confidentiality claims for some data, this information will also be released to the public once the confidentiality assessment finishes.
VERIFICATION STUDIES (ARTICLE 32D)
In cases where EFSA is not convinced by the study data, the Authority may commission studies to verify the evidence submitted – they may have a wider scope than that considered in the original application.
TRANSPARENCY – INFORMATION THAT WILL BE RELEASED TO THE PUBLIC (ARTICLE 38)
EFSA will release to the public (with the exception of information granted confidential status; non-exhaustive list):
- Scientific data and studies, including supplementary information provided during the evaluation (i.e. during a clock-stop).
- Summary of advice provided to applicants at pre-submission phase (after the application is considered valid).
- Minority opinions and results of public consultations.
This information will be available on the EFSA website in an electronic format that can be downloaded, printed & searched through.
CONFIDENTIALITY ASSESSMENT (ARTICLE 39 OF THE TRANSPARENCY REGULATION AND ANNEX I OF THE PRACTICAL ARRANGEMENTS6)
An important novelty introduced by the Transparency Regulation is the change of the authority in charge of assessing the confidentiality requests and providing the final decision. So far, confidentiality has been assessed by the EC, but for dossiers submitted after the 27th March 2021 it will be EFSA. Upon request, EFSA may grant confidential treatment only to (based on proper justifications provided by the applicant):
- Manufacturing processes and analytical methods
- Technical and industrial specifications
- Study plans for efficacy studies of a feed additive – aims of intended use
- Impurity specifications – except those that may have an adverse effect
- Detailed description of starting substance/preparations used for manufacturing the product (food)
- Detailed description of composition of materials/products in which the substance intended for authorisation will be used (food)
- Analytical information on variability, stability of individual production batches (food)
- DNA sequence information, except for sequences used for the purpose of detection, identification and quantification of the transformation event (genetically modified food and feed)
- Breeding patterns and strategies (genetically modified food and feed)
- Commercial links between a producer or importer and the applicant or the authorisation holder, where applicable
- Commercial information revealing sourcing, market shares or business strategy of the applicant
Another remarkable change of this regulation is that now the confidentiality requests will be assessed twice – after dossier validation and at the finalisation of the scientific evaluation (i.e., before publication of the EFSA opinion). This means that EFSA can change its original decision on confidentiality and request applicants the release of additional information from the dossier to the public.
PROTECTION OF PERSONAL DATA (ARTICLE 39E)
The following information will be publicly available: name and address of the applicant, names of authors of published or publicly available studies supporting the application, and names of all participants and observers in meetings of the Scientific Committee and the Scientific Panels, their working groups and any other ad hoc group meeting. The regulation also establishes that the names and addresses of natural persons involved in testing on vertebrate animals or in obtaining toxicological information will be protected.
STANDARD DATA FORMATS (ARTICLE 39F)
EFSA will draft standard data formats for submission of documents such as dossier templates, replies to the authority, submission of supplementary information. These standard data formats will be available online and will allow the functions search, copy, print, and will comply with EU regulatory requirements.
FACT-FINDING MISSIONS (ARTICLE 61A)
A commission of experts could visit laboratories and CROs involved in performing trials for an application submitted to EFSA with the objective of assessing a) compliance with relevant quality standards, and b) compliance with the notification of studies to the EFSA database. In addition to EU locations, these fact-finding missions could also visit facilities in 3rd countries with agreements with Europe.
WHAT TO DO NEXT?
The Transparency Regulation will be a game changer for the registration of feed and food products in the EU and will require a significant effort from applicants in order to comply with all requirements. It will be particularly important for companies to pay attention to the proper and timely notification of studies, or otherwise they risk a significant delay to the risk assessment process and, therefore, access to market.
- Register on the Connect EFSA Portal: website for notification of studies and request of pre-submission advice. In this EFSA video you will find the instructions for registration depending on your profile – applicant, laboratory/CRO or third party (e.g., consultant)
- Get access to the E-submission Food Chain Platform: web-based application to be used by applicants for creating, submitting, and managing dossiers while interacting with the authorities (e.g., EFSA, EC). In order to access, you need to request an EU Authentication Login account – see instructions here
- When planning studies, make sure the laboratories/CROs you are working with are registered in the Connect EFSA Portal – they will have to co-notify the studies if based in the EU or the EEA.
- Discuss the preparation of confidential and non-confidential versions of the study reports with your laboratory/CRO – do not forget to include justifications for everything you wish to claim as confidential.
- Remember to notify the studies before the start date.
- If you are preparing a renewal dossier, draft the list of intended studies (if new) and request pre-submission advice through the Connect EFSA Portal.
- Check the EFSA Toolkit where you will find useful webinars and documents related to pre-submission activities and the e-submission system
- Check Pen & Tec´s YouTube Channel for more useful resources related to the implementation of the Transparency Regulation in the EU
 By Eliana Henriquez-Rodriguez, PHD , Regulatory Affairs Manager
By Eliana Henriquez-Rodriguez, PHD , Regulatory Affairs Manager
Pen & Tec Consulting
REFERENCES
- Regulation (EU) 2019/1381 of the European Parliament and of The Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC. OJ L231, 1-28 (6/09/2019).
- European Commission: Transparency and sustainability of the EU risk assessment in the food chain https://ec.europa.eu/food/safety/general_food_law/transparency-and-sustainability-eu-risk-assessment-food-chain_en (last checked on Jan.14th 2021)
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L31, 1-24 (1/02/2002).
- EFSA. Decision laying down the practical arrangements on pre-submission phase and public consultations. https://www.efsa.europa.eu/en/corporate/pub/tr-practical-arrangements?utm_medium=email&utm_source=ampl&utm_campaign=trec01 (2020)
- Connect.EFSA. EFSA portal for pre-submission activities. https://connect.efsa.europa.eu/RM/s/login/ (2021)
- EFSA. Decision of the Executive Director of the European Food Safety Authority Laying down practical arrangements concerning transparency and confidentiality. https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/210111-PAs-transparency-and-confidentiality.pdf (2021)
