Biopesticides and biostimulants provide opportunity for sustainable agriculture, but how are they regulated in the EU?
Posted 5 May, 2020
Agriculture is facing one of its biggest challenges in history; with a rapidly growing world population, which is expected to reach 9.8 billion people by 2050, and a global decrease in arable land due to climate change, soil erosion and intensive monoculture farming, more food will have to be produced on less arable land. In order to achieve that, the yield gap of arable crops has to be reduced as much as possible. The yield gap can be defined as the difference between the maximum yield potential of a crop and the average farmers’ yield over a specified area and period. Pesticides, fertilisers, high yielding crop varieties obtained by traditional breeding or genetic engineering, among other technologies, all play a role in reducing that yield gap. Pesticides contribute to reducing the yield gap by targeting specific plant pests and/or diseases. Pesticide use therefore, plays an important role in optimising crop yield, which will lead to more production on less arable land. Pesticides include herbicides, fungicides and insecticides.

Although pesticide use has beneficial effects on the productivity of agriculture, the intensive use of chemicals leads to a negative impact on human health and the environment. Academia and industry are therefore looking for various efficacious biological alternatives to reduce the use of chemical pesticides. Biopesticides, in contrast to their synthetic counterparts, contain naturally occurring substances that control specific pests by non-toxic mechanisms. Biopesticides are usually inherently less toxic than conventional chemical pesticides. In contrast to chemical pesticides, biopesticides generally have a narrow spectrum, often targeting one specific pest and closely related organisms. Chemical pesticides, on the contrary, may affect non-target organisms due to their broad-spectrum mode of action, leading to harmful effects to the environment. Moreover, biopesticides often have lower exposures due to their ability to be effective in very small quantities. Biopesticides often decompose more quickly as well, avoiding pollution issues that conventional pesticides can cause.
Biostimulants are another category of biologicals in agriculture that can help to increase crop yield. In contrast to biopesticides, biostimulants do not have any direct actions against pests or disease but act on the plant’s vigour. They can be seen as an extra aid for the plant to thrive and are complementary to crop nutrition and crop protection. Plant biostimulants include diverse formulations of compounds, substances and micro-organisms that are applied to plants, seeds or soils to improve crop vigour, yields and quality by enhancing nutrient uptake, nutrient efficiency and tolerance of abiotic stresses.
Active ingredients used as feed additives, cosmetics, pharmaceuticals may also be effective as biopesticides and biostimulants depending on their mode of action. Algal extracts, micro-organisms, microbial extracts or plant extracts may show beneficial effects that can be exploited for use as a biostimulant and/or a biopesticide. For example, companies already producing specific micro-organisms for use as a feed additives, might be interested in expanding the use of their product, and look into registering their micro-organism as either a biostimulant or a biopesticide as much of the safety data will already be on file. Developing a suitable formulation to optimise the product’s efficacy in the plant as either a foliar spray, seed treatment, or soil application, would however be the most challenging part.
Biopesticides regulatory framework in the EU
Plant protection products (PPPs) include both conventional synthetic pesticides and biopesticides, protecting crops or desirable plants. The core legislation regulating the authorisation for PPPs in the EU is Regulation (EU) No 1107/2009. PPPs contain at least one active ingredient, which can be any chemical, plant extract, pheromone or micro-organism that has action against pests. PPPs are defined under Article 2 of Regulation (EU) No 1107/2009 as products that either protect plants against pests or diseases, influence the life processes of plants such as substances influencing their growth (other than as a nutrient or a plant biostimulant), preserve plant products or destroy or prevent growth of undesired plants or parts of plants. PPPs may additionally contain other components as well, such as safeners, synergists and co-formulants. Safeners are substances or preparations which are added to a PPP to eliminate or reduce phytotoxic effects of the PPP on certain plants. Synergists are substances or preparations which can give enhanced activity to the active substance(s) in the PPP. Both safeners and synergists are regulated on the same level as active substances according to Regulation (EU) No 1107/2009 in terms of requirements for approval. Substances or preparations that are added to a PPP but are neither safeners nor synergists are considered as co-formulants. Unaccepted co-formulants are listed in Annex III of Regulation (EU) No 1107/2009.
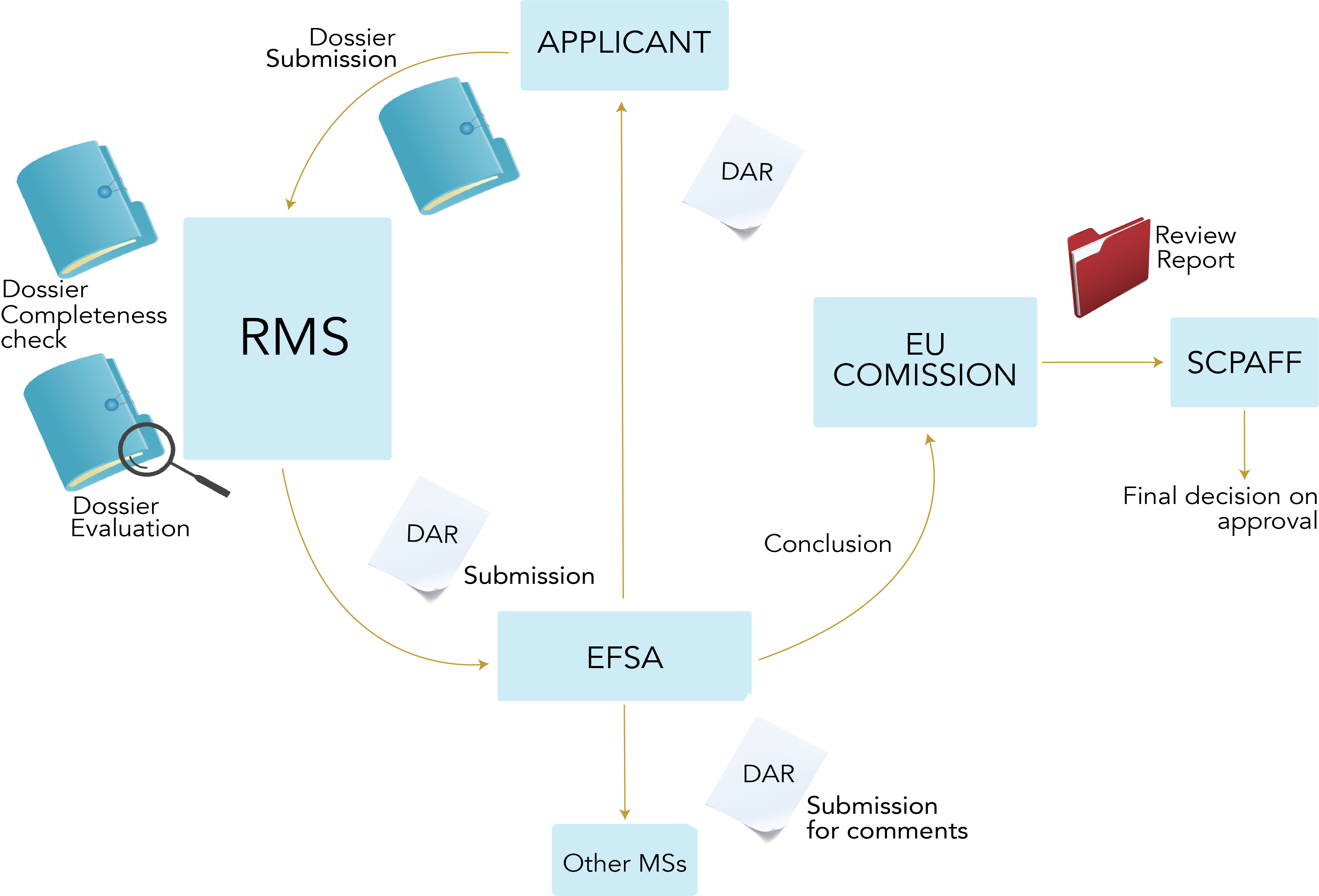
PPPs are evaluated in the EU in a two-step process. Firstly, the active substance is evaluated and approved at EU level and will be included in a list of approved substances which are subject to renewal. Secondly, the formulated product is evaluated and authorised at Member State (MS) level, which is only possible if the active substance has been previously approved at EU level.
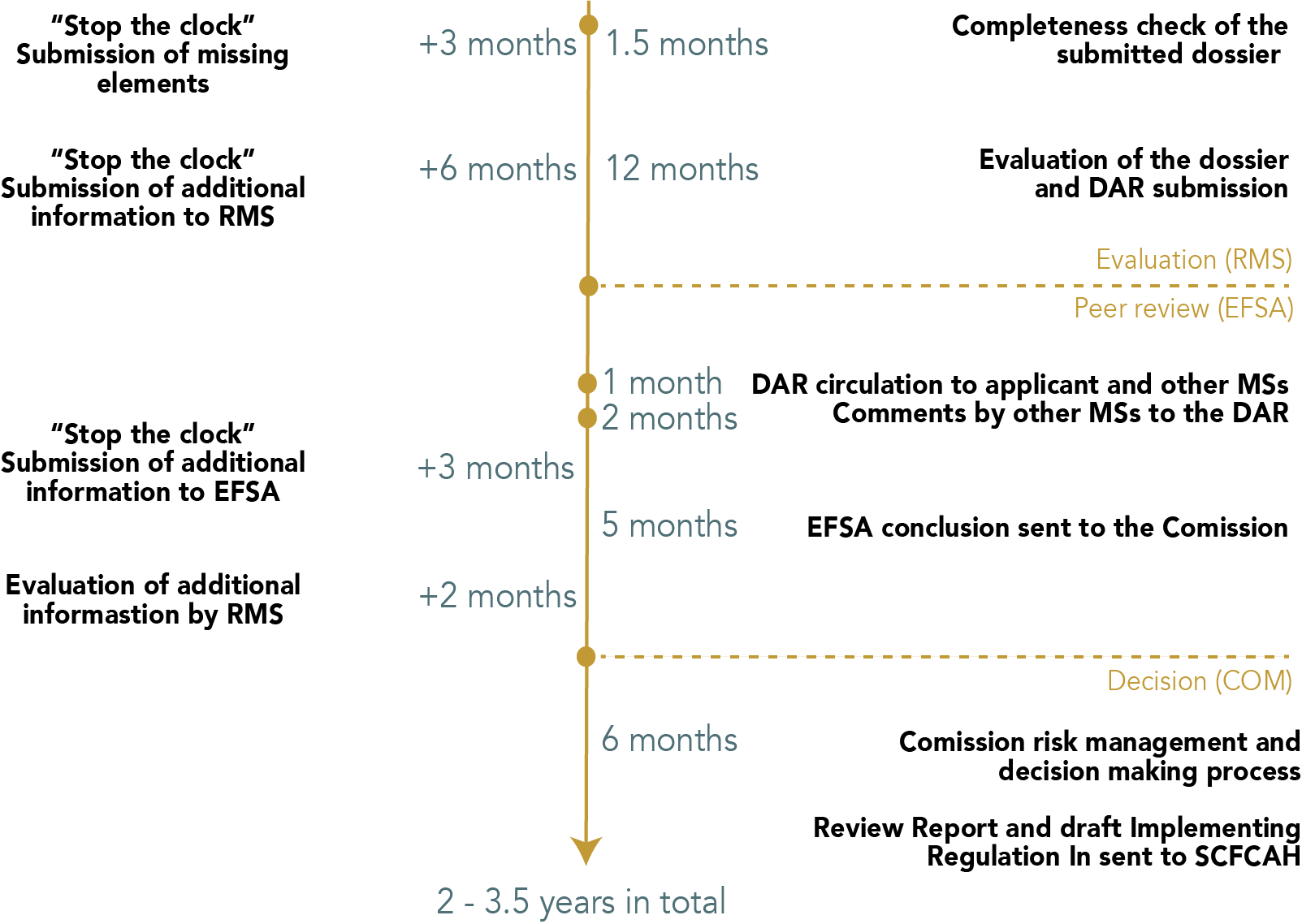
In order to get an approval of an active substance at EU level, an applicant must select a MS that will act as the Rapporteur Member State (RMS) for the active substance. When the chosen MS agrees to do the evaluation, the applicant will send a Draft Assessment Report (DAR) to the RMS. The RMS will evaluate the completeness of the dossier within 1.5 months before starting the scientific evaluation. In case certain information is missing, a clock-stop for a maximum of 3 months is issued to the applicant to submit the missing elements. When the RMS considers the DAR as complete, the scientific evaluation of the dossier can start. The RMS has 12 months to do the scientific evaluation but can issue clock-stops for a maximum of 6 months in total in case additional information is needed from the applicant. Once the RMS has evaluated the active substance, the RMS will submit the DAR to EFSA. EFSA will circulate the DAR to the applicant and to the other MSs. The other MSs have a maximum of 2 months to comment on the DAR. EFSA will peer-review the DAR within 5 months and can issue clock-stops of maximum 3 months to the applicant. The RMS has a maximum of 2 months to evaluate the additional information requested by EFSA. EFSA will issue a scientific opinion based on the assessment of the RMS, which will be sent to the European Commission (EC). The EC has 6 months to do the risk management and issue a final review report to the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF), where a final decision on whether or not to approve the active substance will be taken. The total process of getting an approval for an active substance should take in between 2 and 3.5 years according to the regulation. In practice, these timelines are often much longer. The first approval of an active substance is typically valid for 10 years. The entire process is summarised in Figure 1.
The data requirements for active substances are listed in Regulation (EU) No 283/2013. Chemical active substances should comply with the data requirements in Part A of this regulation and microbial active substances should comply with the data requirements in Part B of this regulation. The data on the active substance should cover:
- Identity of the active substance;
- Physical and chemical properties of the active substance (or biological properties of the micro-organism);
- Further information on the active substance;
- Analytical methods;
- Toxicological and metabolism studies;
- Residues in or on treated products, food and feed;
- Fate and behaviour in the environment;
- Ecotoxicological studies.
The active substance in the PPP that an applicant wants to register, might be the same substance that has already been registered as, e.g. a feed additive. In that case, the applicant will already have several studies on file to cover the specific data requirements for the active substance of the PPP. Those data requirements would include studies for: identity of the active substance, physical and chemical properties of the active substance (or biological properties of the micro-organism), analytical methods, and toxicological and metabolism studies. These studies should be executed with the active substance, and not with a formulated product. Studies executed with the formulated product should be presented in the second step of the registration process.
Once the approval of an active substance has expired, then the active substance is subject to renewal. The process of submission and evaluation of an active substance renewal is similar to the process of submission for the first approval of an active substance, but with shorter timelines. In total, a renewal of an active substance should take 2 to 2.5 years in total, according to the regulation. Also, here, however, timelines are generally much longer in practice.
Once an active substance is approved or renewed on EU level, the formulated product containing the active substance must be authorised or renewed at MS level. In case of a renewal, the applicant must submit the dossier for the product within 3 months from the renewal of the active substance. This strict timeline does not apply for the products containing a new active substance.
In order to get an authorisation for a PPP, the applicant should submit a draft Registration Report (dRR) to each MS where the PPP is intended to be placed on the market. The evaluation of the dRR is carried out on a zonal level in the EU. The EU is divided into three geographical zones, based on climatic conditions:
- Northern zone: Denmark, Estonia, Latvia, Lithuania, Finland, Sweden
- Central zone: Belgium, Czech Republic, Germany, Ireland, Luxembourg, Hungary, Netherlands, Austria, Poland, Romania, Slovenia, Slovakia, (United Kingdom)
- Southern zone: Bulgaria, Greece, Spain, France, Croatia, Italy, Cyprus, Malta, Portugal
The applicant is free to decide which MS will do the zonal evaluation of the dRR in each zone where the product is planned to be marketed. If the MS agrees to do the zonal evaluation, they will function as the zonal Rapporteur Member State (zRMS). This means that, in each zone, one MS will act on behalf of the other MSs in that zone and evaluate the dossier. After reception of the dRR, the zRMS will start with a check for completeness of the dossier. In case the dRR is considered as incomplete, the zRMS can issue a clock-stop to the applicant. Once the dossier is considered as complete, the zRMS will start the scientific evaluation of the dossier. The zRMS can issue clock-stops in case additional information is needed from the applicant during the evaluation. Once the zRMS has finished their evaluation, they will circulate their decision to all other concerned Member States (cMSs) involved in the zonal evaluation. Each cMS is able to comment on the decision of the zRMS. Once the Registration Report (RR) has been finalised, all involved MSs will individually make a decision on the authorisation of the PPP. A MS can grant either a full authorisation, an authorisation restricted to some crops, or reject the authorisation. The evaluation of a new product should take about 12 – 22 months and the evaluation of a product renewal should take about 6 – 9 months. In reality, these timelines are often longer.
Regulation (EU) No 284/2013 lists the data requirements which should be generated for all new products. The dRR submitted to each MS consist of three parts:
- Part A: risk management specific for each MS, containing a summary of the risk assessment in Par B of the dRR;
- Part B: risk assessment containing:
- Part B0: Regulatory context;
- Part B1, B2 and B4: Physical and chemical properties;
- Part B3: Efficacy;
- Part B5: Analytical methods;
- Part B6: Toxicology;
- Part B7: Residues;
- Part B8: Environmental fate;
- Part B9: Ecotoxicology;
- Part B10: Metabolite relevance;
- Part C: Confidential information;
- National addendum required for a specific MS, with special requirements covering environmental fate and ecotoxicology
Once the registration of a PPP is in place in a certain MS, an applicant can apply for Mutual Recognition (MR) in another MS, based on the authorisation of a product in the first MS. The process of MR is much quicker and takes 120 days according to the regulation. The approval of the product will inherently be a copy-paste of the approval in the first MS, including the uses of the product. The product will carry the same label as in the MS of the first approval. The zonal requirements for the products should apply as well, meaning that it is not possible to get an approval in one MS and get approval for the same product in all other MSs based on the MR principle.
Biostimulant regulatory framework in the EU
Biostimulants are regulated very differently from biopesticides in the EU. Products that fall under the definition of a PPP under Article 2 of Regulation (EU) No 1107/2009 are not considered a biostimulant and are excluded from the scope of this regulation. It is important to note that there is a thin line between what is considered as a PPP and what is considered as a biostimulant. According to their definition, PPPs can also be products influencing the life processes of plants, such as substances influencing their growth, other than as a nutrient or a plant biostimulant. These types of PPPs are also known as Plant Growth Regulators (PGRs). PGRs generally interfere with the hormone function in plants. Plant biostimulants have different modes of action. Article 3 of Regulation (EU) No 1107/2009 defines a plant biostimulant as a product stimulating plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: nutrient use efficiency, tolerance to abiotic stress, quality traits, availability of confined nutrients in soil or rhizosphere.
Currently, products qualifying as biostimulants have to find their way to the EU market through the national legislations in each MS. In most MSs, biostimulants can find their way to the market through the national fertilizer legislation.  The different national fertiliser legislations often do not define the term biostimulants, which leaves the categorisation of the product in the national legislation up for interpretation. Because each country has its own set of rules, the data requirements for the dossier of a biostimulant and timelines involved for the evaluation can greatly differ per MS. However, timelines to obtain an authorisation of a biostimulant are often much shorter than those for biopesticides. In some MSs, biostimulants do not fall under any legislation, meaning that the biostimulant can be marketed immediately, under the condition that they are safe for human health and the environment. The responsibility in case incidents happen with the product lays completely in hands of the company commercialising the product.
The different national fertiliser legislations often do not define the term biostimulants, which leaves the categorisation of the product in the national legislation up for interpretation. Because each country has its own set of rules, the data requirements for the dossier of a biostimulant and timelines involved for the evaluation can greatly differ per MS. However, timelines to obtain an authorisation of a biostimulant are often much shorter than those for biopesticides. In some MSs, biostimulants do not fall under any legislation, meaning that the biostimulant can be marketed immediately, under the condition that they are safe for human health and the environment. The responsibility in case incidents happen with the product lays completely in hands of the company commercialising the product.
Last year, the EU has published a new Regulation, which lays down the rules on EU fertilising products. This regulation, Regulation (EU) No 2019/1009, covers biostimulants and entered into force on 16 July 2019. The Regulation will be applied from 16 July 2022. Regulation (EU) No 2019/1009 will not replace the national frameworks, meaning that from July 2022, the applicant will be able to decide whether they want to pursue a European approval for their biostimulant product or obtain an approval through the national legislations in the MSs.
Conclusions
Biostimulants and biopesticides are two categories of biological products that are currently advancing as a response to reduce the amount of chemicals used in agriculture. These products can be very similar in terms of composition. However, both types of products are regulated very differently in the EU.
Biopesticides are regulated on EU level and fall under the scope of Regulation (EU) No 1107/2009 regulating PPPs. They are evaluated in a two-step process. The active substance is first evaluated and approved at EU level. The evaluation is carried out by an RMS appointed by the applicant. Once an approval is in place, then the formulated product is evaluated and authorised at MS level through a zonal evaluation. A zRMS appointed by the applicant will do the evaluation of behalf of the other MSs in the same geographical zone. A very exhaustive data package has to be submitted and associated timelines can be very long before a PPP can enter the European market.
No EU regulatory framework is currently applicable for biostimulants. Regulation (EU) No 2019/1009, which covers biostimulant approvals, recently entered into force and will be applicable as of 16 July 2022. Until then, biostimulants are generally regulated under the national fertiliser legislation of a specific MS. However, this varies from MS to MS. The data package required to get an approval for a biostimulant, and the timelines associated with the evaluation, also vary from MS to MS. Overall, costs and timelines associated with biostimulant dossiers in MSs are far lower than those for biopesticide registrations.
List of acronyms
- cMS: concerned Member State
- DAR: Draft Assessment Report
- EC: European Commission
- MR: Mutual Recognition
- MS: Member State
- PPP: Plant Protection Product
- RMS: Rapporteur Member State
- SCoPAFF: Standing Committee on Plants, Animals, Food and Feed
- cMS: concerned Member State
- dRR: draft Registration Report
- zRMS: zonal Rapporteur Member State
Sources:
- https://ec.europa.eu/food/plant/pesticides_en
- https://www.europarl.europa.eu/cmsdata/185760/EPRS_IDA(2019)634416_EN.pdf
- https://www.epa.gov/ingredients-used-pesticide-products/what-are-biopesticides
- http://www.biostimulants.eu/about/what-are-biostimulants-benefits/
- https://www.ecpa.eu/sites/default/files/7450_Registration%20brochure_3.pdf
- http://www.fao.org/3/a-i4695e.pdf
- https://plant-protection.net/fileadmin/Tagungen/PPPHE/2017/Documents/3_3_Makulla_2017-12-14.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6155630/
 By Hannes Malfroy, Regulatory Affairs Associate
By Hannes Malfroy, Regulatory Affairs Associate
Pen & Tec Consulting